Ligand Binding to a Receptor Kinase Results in
This indicates that the binding of signaling molecules to activated EGFRs results in. Ligand binding induced the formation of receptor oligomers which were found in both the plasma membrane and intracellular structures.
Ligands Receptors Article Khan Academy
How does this mutation influence RAS activity.

. Cell-surface receptors are membrane-anchored proteins that bind to ligands on the outside surface of the cell. However many receptors involve three or more cell surface components and the effect of this. View Test Prep - CHAPTER 9 from BIOLOGY 2107 at Georgia State University.
Given that ligand binding is essential for the rapid internalization of epidermal growth factor receptor EGFR the events induced by ligand binding probably contribute to the regulation of EGFR internalization. Ligand binding induces a conformational change in the ectodomain leading to the reorientation of the intracellular kinase domains resulting in the activation of the asymmetric kinase dimer. Through a still unclear mechanism the ECD dimer communicates with the intracellular fragments of the receptor.
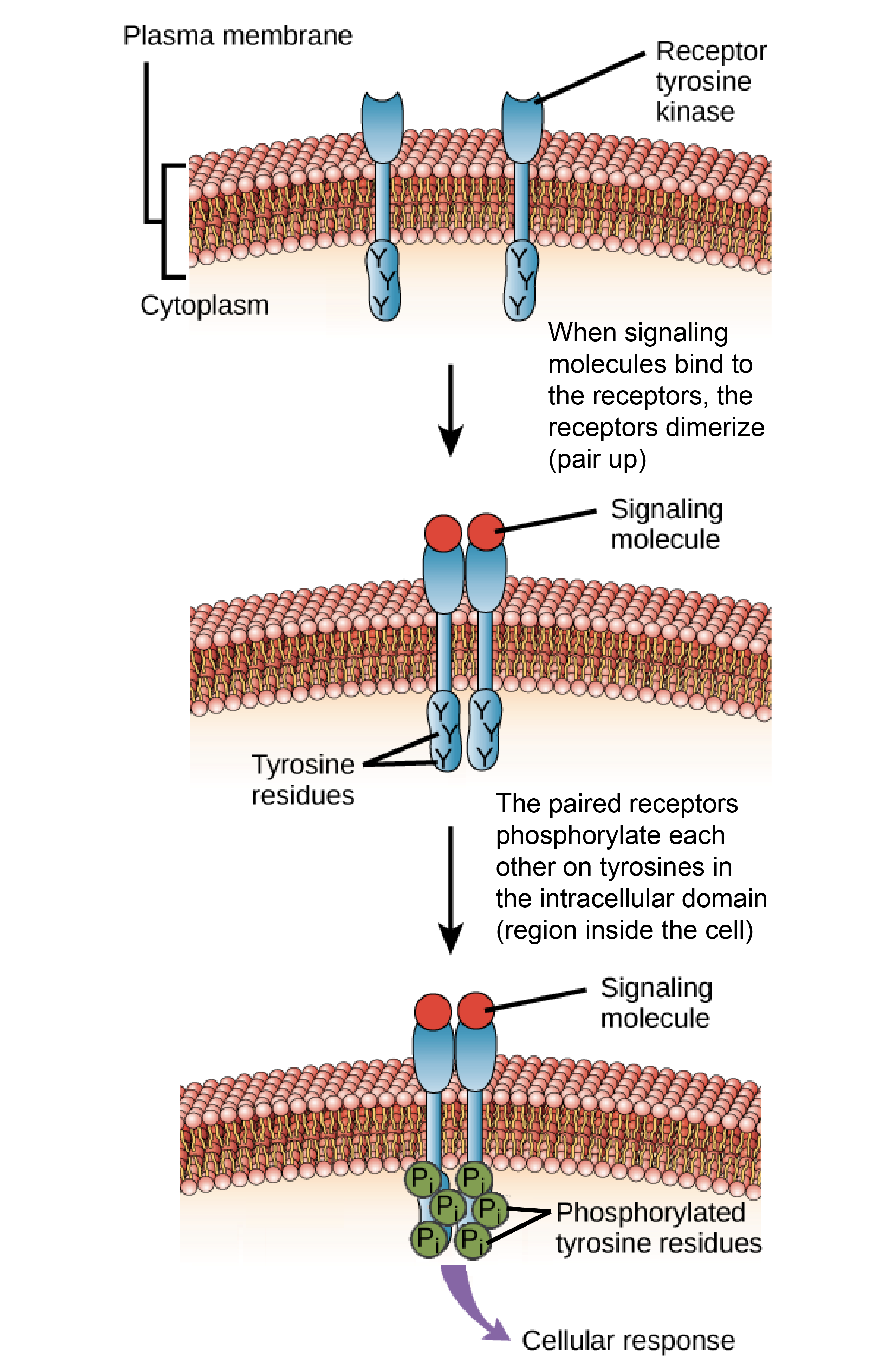
In the canonical model of HER receptor activation ligand binding to the ECDs results in receptor dimerization by promoting an extended conformation of the ECD 16 17 18 19. A change in gene expression resulting in cell division. CHAPTER 9 1Ligand binding to a receptor kinase results in.
Ligand interactions with RTKs induce autophosphorylation receptor endocytosis and activation of downstream signaling such as signaling pathways involving the kinases AKT and extracellular signalregulated kinase 12 ERK 12 11. Experts are tested by Chegg as specialists in their subject area. So many different kinds of molecules including large hydrophilic or water-loving ones may act as ligands.
Ras- GTP PI3K cytosol P-AKT Ras-GTP activates MAP kinase MAPK which in turn activated ETS. What causes the inactivation of a G protein. Ligand binding to the receptor domain regulates the ratio of kinase to phosphatase activities of the signaling domain of the hybrid Escherichia coli transmembrane receptor Taz1 J Mol Biol.
Here we describe a signaling pathway whereby LRP1 transactivates Trk receptors. Receptor tyrosine kinases are the high-affinity cell surface receptors for many polypeptide growth factors cytokines and hormones. Ligand binding to a receptor kinase results in.
Human EGFR can be activated by seven related ligands that generate different signaling outputs from the receptor. Of the 90 unique tyrosine kinase genes identified in the human genome 58 encode receptor tyrosine kinase proteins. Increased kinase activity through autophosphorylation.
What is the end result of activation of the MAP kinase pathway. As signaling proceeds activated receptors will bind to phosphotyrosine-binding proteins such as actin Cbl and Grb2 resulting in the oligomerization of the EGFR. What mutation is commonly found in RAS genes in cancer cells.
RhoA-GTP dissociates from RhoA-GDPi allowing RhoA to bind downstream. All of these choices are correct. The results indicate that the binding of soluble Cross-linking Experiments ligands to the HS chains induces the cell surface ag- gregation of HSPGs over the actin cytoskeleton and Confluent human fibroblasts were washed three times in cold PBS before labeling the plasma membrane proteins with 05 mCi 1251I that the ligand-HPSG complexes are.
Signaling by transmembrane receptor kinases which are composed of an extracellular ligand-binding domain a single transmembrane helix and an intracellular kinase domain is fundamental to. These events include receptor dimerization activation of intrinsic tyrosine kinase activity and autophosphorylation. In PC12 cells and neurons binding of two structurally distinct ligands to LRP1 activated Src family kinases which then activated Trk receptors as measured by Trk receptor tyrosine phosphorylation the activity of two downstream kinases Akt and extracellular signalregulated kinase 1 and 2 and neurite outgrowth assays.
Cytokine and growth factor receptors are activated when the soluble growth factor binds and alters the protein-protein interactions between receptor components on the cell surface triggering intracellular receptor phosphorylation and downstream signaling. Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role. To date no ligand has been identified for ALK that meets these criteria 6 10.
In this type of signaling the ligand does not need to cross the plasma membrane. FGF ligand FGF receptor FGFR Plasma membrane Active FGFR converts Ras from the inactive GDP-bound form not shown to the active GTP- bound form. Binding of cytoplasmic signaling molecules.
Ligand binding to G-protein coupled receptors results in conversion of RhoA-GTP to RhoA-GDP. All of these answer options are correct. The two adjacent tyrosine kinase domains phosphorylate one another to tyrosine residues autophosphorylation.
How does ligand binding to a receptor tyrosine kinase result in the activation of RAS. P-MAPK NFKB ETS G1-Cyclin expression PROLIFERATION NFKB TRAF1 expression SURVIVAL nucleus ETS Active ETS turns. Binding of tissue-type plasminogen activator or α 2-macroglobulin α 2 M to LRP1 resulted in Src family kinase SFK activation and SFK-dependent Trk receptor transactivation in PC12 cells and neurons.
All of these answer options are correct. Ligand-induced oligo-merization required tyrosine kinase activity and nine different tyrosine kinase substrate residues. Ligand binding to the C-terminal ligand binding domain is an important regulatory event in AR function resulting in a conformational change that disrupts an intramolecular interaction between the amino terminus and the carboxy terminus initiates posttranslational modifications dissociates the AR from heat shock proteins HSPs and results in translocation of the active.
Ligand binding to the extracellular domain results in receptor dimerization. Who are the experts. Trk receptor transactivation was necessary for activation of Akt and extracellular.
Ligand binding to a receptor kinase results in. Lemmon et al 2014 it cannot explain how the extracellular. While the classical mechanism for receptor tyrosine kinase activation ligand-induced dimerization is essential for all these ligands to activate the receptor Kovacs et al 2015.
Phosphorylation of the cytoplasmic domain of the receptor.

Schematic Representation Of Receptor Tyrosine Kinase And Downstream Download Scientific Diagram

Upon Ligand Binding Receptor Tyrosine Kinases Rtks Dimerize And Download Scientific Diagram

Upon Ligand Binding Receptor Tyrosine Kinases Rtks Dimerize And Download Scientific Diagram
Comments
Post a Comment